Answer: The molar mass of element X is 35.5 g/mol
Step-by-step explanation:
We are given:
A chemical compound having formula
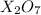
1 mole of this compound contains 2 moles of element X and 7 moles of oxygen
Mass of oxygen atoms = 33.6 grams
Mass of sample = 54.9 g
Mass of X atoms = (54.9 - 33.6)g = 21.3 g
We know that:
Molar mass of oxygen = 16 g/mol
To calculate the molar mass of element X, we use unitary method:
33.6 g of oxygen combines with 21.3 g of element X.
So,
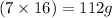 of oxygen atom will combine with
of oxygen atom will combine with
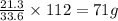 of element X
of element X
From the given formula, 2 moles of element X are present.
So, molar mass of element X =
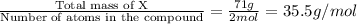
Hence, the molar mass of element X is 35.5 g/mol