Step-by-step explanation:
The given data is as follows.
Volume = 65.5
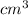
Temperature =
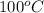
Pressure = 760 torr or 1 atm
As we know that q =

where, m = mass
 = latent heat of fusion
= latent heat of fusion
As density of water is 1.0 g/ml. Hence, mass of water in liquid state is calculated as follows.
mass = density × volume
=
 (as 1 ml = 1
(as 1 ml = 1
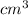 )
)
= 65.5 g
As heat of fusion of ice is 333.55 J/g. Hence, calculate the heat energy as follows.
q =

=
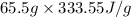
= 21847.52 J
And, heat of vaporization of water is 2257 J/g.
Also, q =
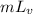
where,
 = heat of vaporization
= heat of vaporization
Therefore, we will calculate the mass of steam as follows.
q =
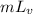
21847.52 J =

m = 9.68 g
It is known that density of steam water is 0.0006 g/ml. Hence, calculate the volume of steam as follows.
Volume =
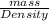
=
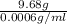
= 16133.33 ml
or, = 16.13 L (as 1 L = 1000 ml)
Thus, we can conclude that 9.68 g of steam condenses will release the same amount of energy as 65.5 g of freezing liquid water and its volume is 16.13 L.