Answer:
The balanced chemical equation can written be as:
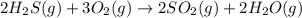
Step-by-step explanation:
Dihydrogen sulfide =

Oxygen gas =

Sulfur dioxide =

Water vapor =

The balanced chemical equation can written be as:
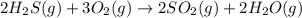
According to reaction , 2 moles of hydrogen sulfide reacts with 3 moles of oxygen gas to give to 2 moles of sulfur dioxide and 2 moles of water vapors.