Answer: Option (b) is the correct answer.
Step-by-step explanation:
Atomic mass means the sum of total number of protons and neutrons present in an atom. On the other hand, atomic number is only the sum of total number of protons present in an atom.
Generally, when we write symbol of a neutral element denoting its atomic mass and number then atomic mass is placed on the top left corner and atomic number is written on the bottom left corner.
For example,
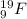 shows atomic mass of fluorine is 19 and its atomic number is 9.
shows atomic mass of fluorine is 19 and its atomic number is 9.
Thus, we can conclude that atomic mass is determined by the number of protons plus the number of neutrons.