Answer:
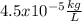
Step-by-step explanation:
Hello!
In this case, since the density in the international system of units is given in terms of kg for the mass and L for the volume, we need to perform a process of units conversions from mg and dL to kg and L as show below:
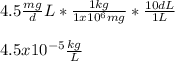
Best regards!