Answer:
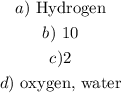
Step-by-step explanation:
a) Here, we want to get the limiting reactant
To get this, we only consider the reactants
This is the left side that contains only hydrogen and oxygen
When we talk of the limiting reactant, we refer to that reactant that would be used up in the formation of the product
Let us take a look at the chamber
We have 10 molecules of hydrogen and 7 molecules of oxygen
To form a molecule of water, we have to use a full molecule of hydrogen and half molecule of oxygen
So, the total number of oxygen present is 7 * 2 = 14
For the hydrogen, we only have 10
14 will outlive 10, so we are left with oxygen
Since hydrogen will be used up and we still have oxygen, then hydrogen is the limiting reactant as it determines the number of molecules of water that will be formed
b) The number of molecules of water formed is the number of hydrogen molecules present. From the count, we have this as 10. That means the number of molecules of water formed is 10
c) From the question, out of the total 14, 10 will be used, remaining 4
A pair makes a molecule. Thus,we have 2 molecules of oxygen left
d) From the question,we know that hydrogen will be used up while oxygen is left. Water is also formed. So we have oxygen and water left in the chamber