Answer:
6.19 L
Explanation:
We are given that
Number of moles of argon gas=0.8 moles
Pressure =P=5.3 atm
Temperature =T=227 degree Celsius=

We have to find the volume of the container needed to store the argon gas.
We know that
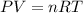
Where R=0.082051 L atm/ mol k
Substitute the values in the given formula


Hence, the volume of container needed to store the gas=6.19 L