Answer:
5.26%
Step-by-step explanation:
Given parameters:
Mass of calcium chloride = 1g
Mass of Water and calcium chloride = 19g
Unknown:
Mass percent of calcium chloride in the water solution = ?
Solution:
To solve this problem, we express the given mass of the calcium chloride as a percentage of the solution.
Mass percent of calcium chloride =
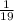 x 100 = 5.26%
x 100 = 5.26%
The mass percentage of calcium chloride is 5.26%