Answer:
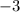
Explanations:
Here, we want to get the charge of the given ion
This is particularly dictated by the charge balance between the electrons and the protons
The electrons are negatively charged while the protons are positively charged
They both have same charge value of 1 albeit electrons are -1 and protons are +1
For 54 protons,we have +54 ,for 57 electrons,we have -57
The balance thus becomes:
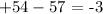
Thus, the charge of the ion is -3