Answer:
5%
Step-by-step explanation:
Given parameters:
Mass of calcium chloride = 5g
Mass of water = 95g
Unknown:
Mass percent of calcium chloride in solution = ?
Solution:
The mass percentage is the mass of the solute component divided by the total mass of the mixture multiplied by 100%.
So;
Mass of mixture = mass of calcium chloride + mass of water = 95g + 5g = 100g
Mass percentage of calcium chloride =
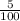 x 100 = 5%
x 100 = 5%