Answer:
the final temperature of the tea is 7.39⁰C.
Step-by-step explanation:
Given;
mass of the tea, m = 375 g
specific heat capacity of the tea, C = 4.184 JJ/g°C
initial temperature of the tea, t₁ = 95°C
the final temperature of the tea, t₂ = ?
Energy lost by the refrigerator, Q = 137,460 J
The energy lost by the refrigerator is given by the following formula;
-Q = mc(t₂ - t₁)
-137,460 =375 x 4.184(t₂ - 95°C)
-137,460 = 1569(t₂ - 95°C)
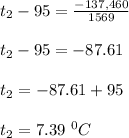
Therefore, the final temperature of the tea is 7.39⁰C.