Answer:
The correct answer is option A.
Step-by-step explanation:
Total pressure of mixture = p = 378 kPa
Partial pressure of the oxygen gas =
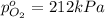
Partial pressure of the hydrogen gas =
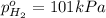
Partial pressure of the nitrogen gas =
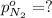
As we know that total pressure is sum of partial pressures exerted by all the individual gases.
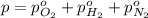
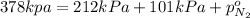
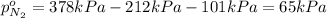
65 kPa the partial pressure exerted by nitrogen.