Answer: Option (C) is the correct answer.
Step-by-step explanation:
An acid which is able to dissociate completely into ions in water is known as a strong acid.
For example,
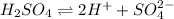
Therefore, sulfuric acid is a strong acid as it dissociates completely into ions in water.
Whereas
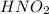 ,
,
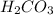 , and
, and
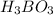 are all weak acids as they dissociates slightly in water as follows.
are all weak acids as they dissociates slightly in water as follows.
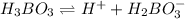

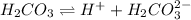
Morover, sufuric acid upon dissociation is giving 2 hydrogen ions and rest all are giving one hydrogen ion.
Thus, we can conclude that out of the given options
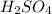 has the largest concentration of hydronium [H3O+].
has the largest concentration of hydronium [H3O+].