Assuming the gas behaves as an ideal gas, since the temperature is maintained constant, we can use the Boyle's Law to answer the question.
The Boyle's Law states that, in these conditions, the pressure and the volume has an inverse proportional relationship, that is, for any pair of pressure P and volume V:
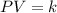
Where k is constant.
In the first situation, we have:
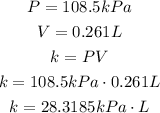
In the second situation, we have the same k, but different P an V:
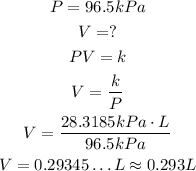
So, the volume the gas occupy is approximately 0.293L.