Answer:
Step-by-step explanation:
Chemically, we can have a reaction between chlorine gas and solid sodium
This reaction is actually explosive and would produce fine powder of sodium chloride
We have the reaction as follows:
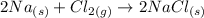
Essentally, what we can deduce from here is that we do not need to add water to the flask. Except for the reason that we would want the sodium chloride solid in the solution form, there is absolutely no reason to add water to the flask as the reaction would proceed normally