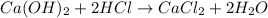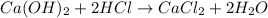
Step-by-step explanation:
First of all, let's try to identify the different substances involved in the reaction:
- calcium hydroxide: calcium is the element
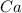 , while hydroxide corresponds to the ion
, while hydroxide corresponds to the ion
 . Calcium has 2 valence electrons, so the calcium ion is
. Calcium has 2 valence electrons, so the calcium ion is
 , and since the ion
, and since the ion
 has only one negative charge, we need two of them to combine with the calcium ion, therefore:
has only one negative charge, we need two of them to combine with the calcium ion, therefore:

- hydrochloric acid: this is an acid, so with one ion of hydrogen
 , and the other element is the chlorine, which in the ion form is
, and the other element is the chlorine, which in the ion form is
 , so the hydrochloric acid is
, so the hydrochloric acid is

- calcium chloride: this is the combination of the calcium ion,
 , with the chlorine ion,
, with the chlorine ion,
 , so the compound is
, so the compound is

- Water: this is given by
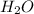
so, the reaction is
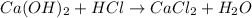
However, this reaction is not balanced yet: in fact, we have 2 atoms of oxygen on the left and only one on the right. Moreover, we have 3 atoms of hydrogen on the left and only 2 on the right. Therefore, in order to balance it, we must write: