The mass of hydrogen is 5.5g.
1st) We are going to assume that the density of water is 1g/ml, so using the density formula and replacing the volume on it, we can calculate the mass of water:
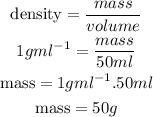
Now we know that the mass of water is 50g.
2nd) The mass of water calculated (50g) represents the 100% of the mass.
With the percent of hydrogen, we can calculate the mass of hydrogen:
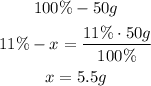
If 100% represents 50g of water, then the 11% of hydrogen in the water represents 5.5g.
So, the mass of hydrogen in 50ml of water is 5.5g.