Step 1 - Understanding the relation between pressure, volume and temperature
The state of a gas can be described by three main variables: pressure (p), volume (V) and temperature (T). They are also related to the number of moles through the following equation:
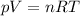
In this equation, R represents the universal gas constant. We can use this equation to understand how volume, temperature and number of moles will affect pressure.
Step 2 - Isolating pressure
Let's isolate p in the previous formula:
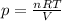
We can see that, according to this relation, p is proportional to the temperature (T), proportional to the number of moles (n) and inversely proportional to the volume (V).
Therefore, increasing the temperature and the number of moles would increase the pressure, whereas increasing the volume would decrease the pressure.
Step 3 - Solving the exercise
Note we are looking for the sample in which the pressure is the lowest possible. In other words, we are looking for the lowest temperature, lowest number of moles and largest volume.
The largest volume possible is 40ml. The lowest temperature possible is 50°C. The lowest possible number of molecules is 6.
These conditions are met in sample A. Therefore, the correct answer is 40ml, 50°C, 6 molecules.
Answer: 40ml, 50°C, 6 molecules