Answer:
0.68L
Explanations
The formula for calculating the molarity of a solution is expressed as:
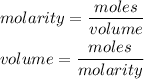
Given the following parameters
Moles of solution = 3.4moles
molarity of solution = 5.0M
Substitute
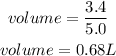
Hence the volume of 3.4 moles in a 5.0 M solution is 0.68L