Answer: The correct option is (A).
Step-by-step explanation:
Neutralization reaction: A chemical reaction which acid is neutralizes by the base and water is produced as a product is known as neutralization reaction.
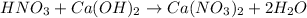
Here in the given reaction, calcium hydroxide being base reacts with nitric acid to produce calcium nitrate and water as a product. So, from the given options the correct answer is of an option (A).