Answer: 43.3 grams
Explanation:-
Molarity of a solution is defined as the number of moles of solute dissolved per Liter of the solution.

Thus
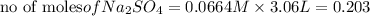
Thus
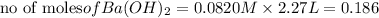
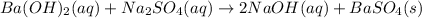
As 1 mole of
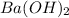 reacts with 1 mole of
reacts with 1 mole of

0.186 moles of
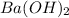 reacts with =
reacts with =
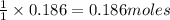 of
of

Thus
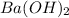 is the limiting reagent and will limit the formation of the products.
is the limiting reagent and will limit the formation of the products.
As 1 mole of
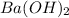 gives with 1 mole of
gives with 1 mole of
 precipitate
precipitate
0.186 moles of
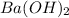 reacts with =
reacts with =
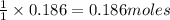 of
of
 precipitate
precipitate
Mass of
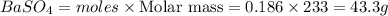
Thus mass of the precipitate formed is 43.3 grams.