Option D: 46.0 g
The balanced chemical reaction is as follows:

The standard temperature and pressure conditions are 273.15 K and 1 atm respectively.
First calculate the number of moles of
 formed at STP,
formed at STP,
Since,
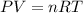
Here, P is pressure, V is volume, n is number of moles, R is gas constant and T is temperature.
Rearranging the equation,

Thus, number of moles of
 gas will be 1 mol.
gas will be 1 mol.
From the chemical reaction, 2 mol of Na gives 1 mol of
 thus, number of moles of Na will be 2 mol.
thus, number of moles of Na will be 2 mol.
Molar mass of Na is 22.99 g/mol thus, mass can be calculated as follows:

Therefore, mass of Na is 46.0 g.