There are 1.99 moles in 1.20x10^24 atoms of copper.
To solve the exercise we have to use the Avogadro's number (6.022x10^23). With the Avogadro's number we know that there are 6.022x10^23 particles in 1 mol, in this case, the particles are atoms of copper.
Now, with a mathematical Rule of Three we can calculate the number of moles in 1.20x10^24 atoms of copper:
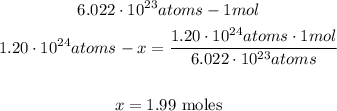
So, there are 1.99 moles in 1.20x10^24 atoms of copper.