Answer: 3.0 moles of water
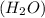 are needed to react with a mole of 3.0 of Na
are needed to react with a mole of 3.0 of Na
Step-by-step explanation:
The balanced chemical reaction of sodium with water is as follows:
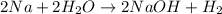
According to stoichiometry:
2 moles of sodium
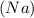 reacts with = 2 moles of water
reacts with = 2 moles of water
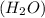
Thus 3.0 moles of sodium
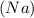 reacts with =
reacts with =
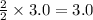 moles of water
moles of water
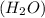
3.0 moles of water
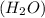 are needed to react with a mole of 3.0 of Na
are needed to react with a mole of 3.0 of Na