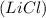Answer: B). lithium and chlorine
Explanation: An ionic bond is formed between a metal and a non-metal by transfer of electrons from metal to non metal.
Electronic configuration of lithium:
![[Li]:31s^22s^1](https://img.qammunity.org/2017/formulas/chemistry/high-school/bhxj2nra1u4t9z7qalhph65xql0on2ykhw.png)
Lithium atom will loose one electron to gain noble gas configuration and form lithium cation with +1 charge.
![[Li^+]=1s^22s^2](https://img.qammunity.org/2017/formulas/chemistry/high-school/3cr5hgw63tkteblbzfc27agnfon2khnx5p.png)
Chlorine atom has oxidation number of -1.
Electronic configuration of chlorine;
![[Cl]=1s^22s^22p^63s^23p^5](https://img.qammunity.org/2017/formulas/biology/high-school/25e9s1l4sq39a62edz9ahmcow0bmjm5m3p.png)
Chlorine atom will gain one electron to gain noble gas configuration and form chloride ion with -1 charge.
![[Cl^-]=1s^22s^22p^63s^23p^6](https://img.qammunity.org/2017/formulas/biology/high-school/s1g64qvi56xdkzq8l15v9iwz8d95ceacxa.png)
 combine with
combine with
 to form lithium chloride
to form lithium chloride