Answer: B) It allows chemists to deal with a large number of atoms.
Step-by-step explanation:
A mole is defined as the amount of substance that contains Avogardro number of the substance.
Avogadro's number is given by
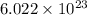 .
.
1 mole of the substance weighs equal to the molecular weight of the substance.
1 mole of the substance occupies 22.4 L of space at standard conditions of temperature and pressure.
Thus mole allows chemists to deal with a large number of atoms.