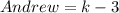Question:
Andrew owns 3 fewer DVDs than Paul. Let K represent the number of Paul's DVDs. Write the expression that can be used to find the number of DVDs that Andrew owns.
Answer:
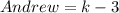
Explanation:
Given
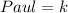
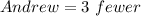
Required
Write an expression for Andrew
3 fewer means that we subtract 3 from Paul's number of DVDs.
i.e.
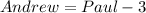
Substitute k for Paul