The molar mass of the carbohydrate : = 304.19 g/mol
Further explanation
Given
6.54 g carbohydrate
102.5 g of water
osmotic pressure of 4.61 atm
T = 20+273=293 K
Required
The molar mass
Solution
General formula:
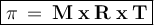
π = osmosis pressure (atm)
M = solution concentration (mol / l)
R = constant = 0.08205 L atm mol-1 K-1
T = Temperature (Kelvin)
Find molarity(M) :
4.61 atm = M . 0.08205 x 293
M = 0.192 mol/L(mol solute per 1 liter solution)
Total mass of solution :
= 6.54 g + 102.5
= 109.04 g
Volume of solution :
= density x mass
= 1.024 g/ml x 109.04 g
= 111.66 ml
= 0.112 L
mol Carbohydrate (solute):
= M x V
= 0.192 x 0.112
= 0.0215 mol
Molar mass of Carbohydrate :
= mass : mol
= 6.54 : 0.0215
= 304.19 g/mol