Answer: The correct answer is decreasing temperature.
Step-by-step explanation:
The relation between pressure, temperature, volume and number of moles of gas is given by the equal known as ideal gas equation:
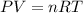
or,

where,
P = Pressure of the gas
V = Volume of the gas
n = Number of moles of gas
R = Gas constant
T = Temperature of gas
From the above relation, it is visible that pressure is directly related to number of moles and temperature of the gas. So, if pressure decreases, the number of moles and temperature of the gas also decreases.
Also, pressure is inversely related to volume of the gas. So, if pressure gets decreased, the volume of the gas must increase.
And, pressure is directly related to number of collisions of the particles. Thus, if the pressure decreases, this means that number of collisions between the particles must also decrease.
Hence, the correct answer is decreasing temperature.