Answer:
The initial temperature of the hot water is
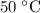 (assuming that no heat was lost to the surroundings.)
(assuming that no heat was lost to the surroundings.)
Step-by-step explanation:
Let
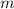 denote the mass of the hot water.
denote the mass of the hot water.
The question states that the mass of the water at
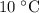 is three times the mass of the hot water. If the mass of the hot water is
is three times the mass of the hot water. If the mass of the hot water is
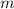 , the mass of the cold water would be
, the mass of the cold water would be
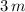 .
.
Let
 denote the specific heat capacity of water. Let
denote the specific heat capacity of water. Let
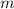 denote the mass of some water. The energy required to change the temperature of that much water by
denote the mass of some water. The energy required to change the temperature of that much water by
 (without state change) would be:
(without state change) would be:
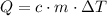 .
.
The temperature change for the cold water was:
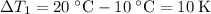 .
.
Energy required to raise the temperature of water with mass
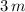 from
from
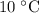 to
to
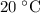 :
:
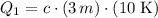 .
.
On the other hand, if the initial temperature of the hot water is
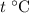 (where
(where
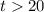 ,) the temperature change would be:
,) the temperature change would be:
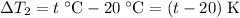 .
.
Calculate the energy change involved:
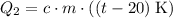 .
.
If no energy was lost to the surroundings,
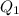 should be equal to
should be equal to
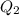 . That is:
. That is:
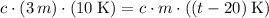 .
.
Simplify and solve for
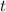 :
:
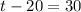 .
.
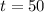 .
.
Therefore, the initial temperature of the hot water would be
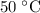 .
.