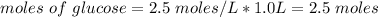Answer:
Moles of glucose = 2.5 moles
Step-by-step explanation:
Given:
Volume of the glucose solution, V = 1.0 L
Concentration or molarity of the solution = 2.5 M
To determine:
The number of moles of glucose in the above solution
Step-by-step explanation:
Concentration of a given solution can be expressed in terms of Molarity which denotes the number of moles of a solute present in a liter volume of a solvent
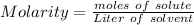
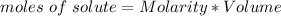
In this case,