Answer:
Scores between 34 and 70 on the fourth test will give Paul a C for the semester.
Explanation:
The average is given by the sum of all scores divided by the number of scores.
In this question:
Sum of all scores: 75 + 80 + 91 + x = 246 + x
Number of tests : 4
Grade of 70:
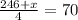
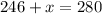
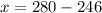
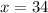
Grade of 79:
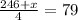
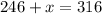
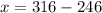
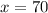
Scores between 34 and 70 on the fourth test will give Paul a C for the semester.