Answer:
Unit rate per loaf of bread is
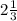 cups of flour.
cups of flour.
Explanation:
It is given that:
Priya uses 7 cups of flour to make 3 loaves of banana bread.
3 loaves = 7 cups
Unit rate per loaf =
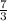
Unit rate per loaf =
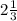 cups
cups
Therefore,
Unit rate per loaf of bread is
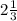 cups of flour.
cups of flour.