Answer:
b. pressure and volume
Step-by-step explanation:
According to boyle's law, which two quantities are inversely proportional?
a. time and pressure
b. pressure and volume
c. volume and temperature
d. pressure and temperature
boyle's law states tat tat tr pressure on a fixed mass of as is inversely proportional to te volume provided tat temperature is kept constant.
mathematically
p
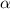 1/V
1/V
P=1/VK.................1
K=PV
comparing between two ratios
P1V1=P2V2
therefore non of the option , except b typifies the boyles law
b. pressure and volume
from equation 1, we can conclude tat as pressure increases, volume decreases. As pressure decreases ,volume increases