Answer:
82.95g of oxygen gas react with 27.0g of acetylene.
Step-by-step explanation:
1st) It is necessary to balance the chemical reaction:
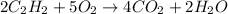
With the chemical reaction we know that 2 moles of C2H2 react with 5 moles of O2 to produce 4 moles of CO2 and 2 moles of water.
2nd) With the molar mass of acetylene and oxygen we can convert the moles to grams:
- Acetylene molar mass (C2H2): 26.04g/mol
- Oxygen molar mass (O2): 32g/mol
- Conversion of C2H2 moles to grams:
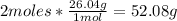
- Conversion of O2 moles to grams:
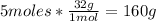
Now we know that 52.08g of acetylene react with 160g of oxygen gas.
3rd) Calculate the grams of oxygen that react with 27.0g of acetylene, using a mathematical rule of three:
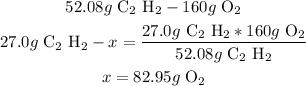
So, 82.95g of oxygen gas react with 27.0g of acetylene.