Answer: the temperature of the gas
Explanation: The ideal gas law has arrived from the combination of four laws:
1) Boyle's Law: This law states that pressure is inversely proportional to the volume of the gas at constant temperature and number of moles.
 (At constant temperature and number of moles)
(At constant temperature and number of moles)
2) Charles' Law: This law states that volume is directly proportional to the temperature of the gas at constant pressure and number of moles.
 (At constant pressure and number of moles)
(At constant pressure and number of moles)
3) Gay-Lussac's Law: This law states that pressure is directly proportional to the temperature of the gas at constant volume and number of moles.
 (At constant volume and number of moles)
(At constant volume and number of moles)
4) Avogadro's Law: This law states that volume is directly proportional to the number of moles of the gas at constant pressure and temperature.
 (At constant temperature and pressure)
(At constant temperature and pressure)
According to ideal gas law,
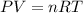
where,
P = pressure of the gas
V = volume of the gas
T = temperature of the gas
n = number of moles of gas
R = Gas constant
Thus to calculate the gas constant we need to know the temperature of gas.