Answer : The energies of the 4s and 4p orbitals in potassium is,

Solution :
First we have to calculate the change in energy.
Formula used :

where,
 = change in energy
= change in energy
h = Planck's constant =
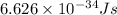
c = speed of light =

 =
=

Now put all the given values in this formula, we get

The difference in energy levels is defined as,

Now we have to calculate the first ionization energy for one atom of potassium.

Now this ionization energy will be equal to the negative of the orbital energy of the electron located in the 4s-orbital.

Now we have to calculate the energy of the 4p orbital in potassium.



Therefore, the energies of the 4s and 4p orbitals in potassium is,
