Answer:
1. b. increase [NH3].
2. d. 0.01.
Step-by-step explanation:
Hello!
In this case, for these equilibrium problems, we proceed as follows:
1. Here, we consider the Le C hatelier 's principle applied to a gas-phase reaction in which the reactants side have more number of moles; therefore, increasing the pressure will increase the products side as it has the fewest number of moles, so the answer is b. increase [NH3].
2. Here, we know that the equilibrium constant of the reverse reaction equals the inverse of the equilibrium constant of the forward reaction, thus, we obtain:
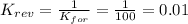
Therefore, the answer is d. 0.01.
Best regards!