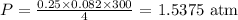Answer:
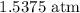
Step-by-step explanation:
Here, we want to get the value of the pressure in atm
Mathematically, we can use the ideal gas equation for this
This is calculated as follows:
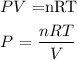
where n is the number of moles which is given as 0.25
R is the molar gas constant which is 0.082
T is the temperature, given as 300K
V is the volume, given as 4L
Substituting these values, we have it that: