Answer:
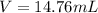
Step-by-step explanation:
Hello!
In this case, since the given 5.00 % (w/v) can also be represented in terms of mass of solute over volume of solution, we need to make sure that the same system of units is used, in this case, the appropriate units for that unit of concentration should be g/mL, and therefore, we first need the mass of citric acid in grams:
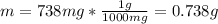
Thus, by using the w/v percent, we obtain the following volume of solution:
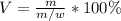
So we plug in to obtain:
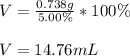
Best regards!