Answer:
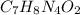
Step-by-step explanation:
Hello!
In this case, since the determination of an empirical formula is covered by first computing the moles of each atom as shown below:
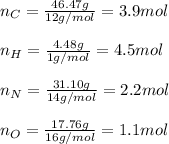
Now, we divide each moles by the fewest moles (those of oxygen), to obtain the subscripts in the empirical formula:
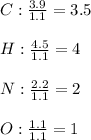
Thus, the empirical formula, taken to the nearest whole subscript is:
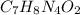
Whose molar mass is 180.16, therefore the empirical formula is the same to the molecular one.
Best regards!