Answer: By using mole concept
Step-by-step explanation:
We are given:
An unknown compound having some number of molecules.
To calculate the number of moles that are contained in a sample, we use mole concept:
According to mole concept:
1 mole of any compound contains
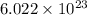 number of molecules.
number of molecules.
Using this relation and applying unitary method, we can easily calculate the number of moles of a compound.
For Example: A sample of water contains
 number of molecules.
number of molecules.
So, by using mole concept:
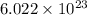 number of molecules are contained in 1 mole of a compound.
number of molecules are contained in 1 mole of a compound.
So,
 number of molecules will be contained in
number of molecules will be contained in
 of water
of water