Answer
b) 0.31 mol/L
Step-by-step explanation
Given that:
Volume of solution = 0.50 L
Mass of sodium chloride = 9.0 g
What to find:
The concentration of the sodium chloride solution.
Step-by-step solution:
The first step is to convert 9.0 g NaCl to moles using the mole formula.
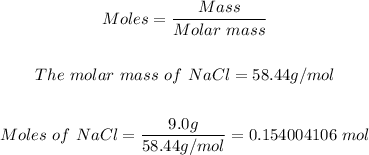
Hence, the concentration of the NaCl solution can be calculated using the molarity formula.
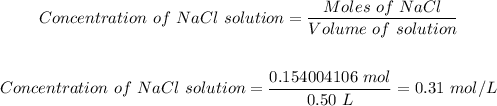
The concentration of a 0.50 L solution containing 9.0 g of sodium chloride is thus b) 0.31 mol/L