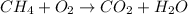Answer : The correct option is A.
Explanation :
As per the question, in a reaction, two reactants (methane and oxygen) should be present on the left side of the right-arrow and two products (carbon dioxide and water) which are formed in the reaction should be present on right side of the right-arrow.
Therefore, the unbalanced chemical reaction will be,