Answer: Nitrogen has the highest ionization energy.
Step-by-step explanation:
Ionization energy is defined as the amount of energy required to remove an electron from an isolated gaseous atom.
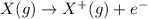
In a periodic table, ionization energy increases as we go from left to right in a period and decreases when we go down in a group.
Group 5A elements are Nitrogen, phosphorous, arsenic, Antimony and Bismuth.
So, the order of ionization energy in the group will be:
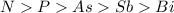
Hence, the nitrogen has the highest ionization energy.