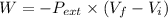Answer:
Following are the solution to this question:
Step-by-step explanation:
The first volume of its blood products analysis is
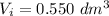 at a temperature of 0° C. In the isothermal expansion of its sample data in a
at a temperature of 0° C. In the isothermal expansion of its sample data in a
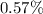 reduction of its size; the actual sample size is thus
reduction of its size; the actual sample size is thus
![V_f = [(0.550) - 0.57 \% \ of (0.550)] dm^3](https://img.qammunity.org/2022/formulas/chemistry/high-school/rkgucy44a1jtsyqay8ent1ww8cau81cwhz.png)
![= [(0.550)-((0.57)/(100)) * (0.550)] \ dm^3 \\\\= [(0.550)-(0.0057) * (0.550)] \ dm^3 \\\\= [0.550-0.003135] \ dm^3 \\\\= 0.546865 \ dm^3](https://img.qammunity.org/2022/formulas/chemistry/high-school/5nv0gn4g3f2mo3dh5reu529shxw3rlrtt5.png)
It is used to persistent the external pressure toward
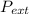 was =
was =
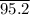 . It is the job completed is, therefore,
. It is the job completed is, therefore,