Answer :
The name of compound
 is, potassium iodide.
is, potassium iodide.
The name of compound
 is, iron (III) oxide.
is, iron (III) oxide.
The name of compound
 is, ammonium phosphate.
is, ammonium phosphate.
Explanation :
Ionic compound : It is a type of compound that is made up of the ions of the two different elements in which one element is a metal and another element is a non-metal.
The rules of ionic compounds is given by:
- Positive ion is written first.
- The negative ion is written next and a suffix is added at the end of the negative ion. The suffix written is '-ide'.
- In case of transition metals, the oxidation state are written in roman numerals in bracket in-front of positive ions.
(1) The given compound
 is an ionic compound. It is made up of two different ions which are potassium ion
is an ionic compound. It is made up of two different ions which are potassium ion
 and iodide ion
and iodide ion
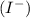 .
.
So, the name of
 is, potassium iodide.
is, potassium iodide.
(2) The given compound
 is an ionic compound. It is made up of two different ions which are iron ion
is an ionic compound. It is made up of two different ions which are iron ion
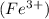 and oxide ion
and oxide ion
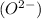 . In this an oxidation state of iron is (+3).
. In this an oxidation state of iron is (+3).
So, the name of
 is, iron (III) oxide.
is, iron (III) oxide.
(3) The given compound
 is an ionic compound. It is made up of two different ions which are ammonium ion
is an ionic compound. It is made up of two different ions which are ammonium ion
 and phosphate ion
and phosphate ion
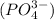 .
.
So, the name of
 is, ammonium phosphate.
is, ammonium phosphate.