Answer:
252 g KOH.
Step-by-step explanation:
a. First, let's see the formula of molarity:
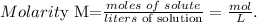
Remember that the volume must be in liters. 1 L equals 1000 mL, so the conversion is:
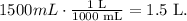
We want to find the grams of solute, so we can find initially the moles of solute, so let's solve for moles of solute and replace the given data:
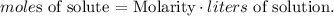
The molarity is 3 M and the volume is 1.5 L:
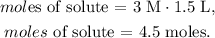
Now that we have this value, we can find its mass by using its molar mass. (The molar mass of KOH is 56 g/mol. You can calculate the molar mass of a compound using the periodic table). The conversion would be:
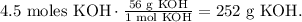
We require 252 g of KOH.