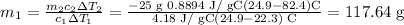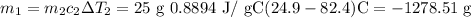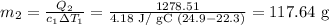Answer
117.64 g
Procedure
Upon inspection, one of the given heat capacities is in J/°C mol; we will convert it to J/g°C
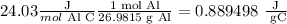
To solve this problem, we will assume that the heat released by the aluminum piece is the same as the heat absorbed by the water.
We have the specific heat formula
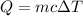
Where
Q=heat energy
m=mass
c=specific heat capacity
Δ T=change in temperature
Then we will compare both equations and consider the variables, with subindex 1 being for water and 2 for aluminum
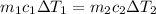
Solving for m1, we have