Answer: The correct option is B.
Explanation: This is an example of radioactive decay and all the radioactive decay processes follow First order of kinetics.
Expression for the half life of first order kinetics is:
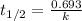
We are given:
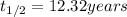
Putting in above equation, we get:
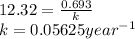
Expression to calculate the amount of sample which is unchanged is:
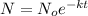
where,
N = Amount left after time t
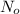 = Initial amount
= Initial amount
k = Rate constant
t = time period
Putting value of k = 0.05625 and t = 24.6 in above equation, we get:
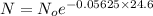
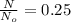
The above fraction is the amount of sample unchanged and that is equal to
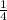
Hence, the correct option is B.